How to Avoid Hydrogen Embrittlement in Steel

FAQs: How to Prevent Hydrogen Embrittlement in Steel and Other Hydrogen Handling Issues
Buddy Damm, Senior Scientist, Swagelok
Hydrogen is one promising solution. Handling hydrogen safely and reliably, from generation to end use, is the key to reaching its fullest potential as a zero-emission fuel source in a wide variety of applications.
However, containing and transferring hydrogen presents some unique challenges. Hydrogen is the first element in the periodic table, consisting of a single positively charged proton and a single negatively charged electron. It is the lightest and among the smallest atoms. In the developing hydrogen economy, hydrogen will need to be handled in liquid form and gaseous form. Hydrogen liquifies at −252.9°C (−423°F) and is about 140 times denser as a liquid compared to the gaseous form. Transporting and storing hydrogen as a liquid is more efficient, but at its point of use, hydrogen is a gas. As a result, two phenomena can impact metals used in hydrogen systems.
- Low-temperature embrittlement: As temperature decreases, metals lose some ductility.
- Hydrogen embrittlement: At ambient temperatures where hydrogen is a gas, atomic hydrogen can diffuse into the metal and cause embrittlement.
Here, embrittlement refers to a reduction in a metal’s ductility and resistance to fracture and fatigue in its service environment compared to its resistance to fracture and fatigue in air and at room temperature. These issues can lead to system failure, resulting in safety risks, increased downtime, and financial loss. As the hydrogen industry continues to expand, addressing these system construction challenges is critical to the fuel’s widespread adoption as a long-term, sustainable fuel solution.
So, how can hydrogen professionals build hydrogen handling systems that last? It is critical to remember that materials matter for hydrogen containment. Fluid systems made with specifically formulated, high-quality stainless steel can better resist the challenges inherent to hydrogen containment. The following FAQs will explain how and what specifiers should be considering in their materials selection for hydrogen containment components.
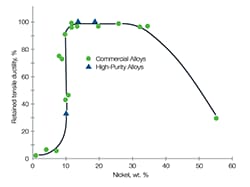
Q: What is low-temperature embrittlement of metal and what materials resist it?

A: Low-temperature embrittlement of metal refers to a reduction in ductility, toughness, or fatigue and fracture resistance as the temperature is decreased. Different types of stainless steel have different levels of resistance to embrittlement-related cracking. See Figure 1 for details. Austenitic stainless steels suffer only minor, low-temperature embrittlement while ferritic steels (low-alloy steels as well as ferritic or duplex stainless steels) are more susceptible. For this reason, austenitic stainless steels are the gold standard for liquid hydrogen systems and is the type of stainless steel to pursue if you are concerned about low-temperature embrittlement.
Q: What is hydrogen embrittlement and what causes it?

A: Hydrogen embrittlement is a form of hydrogen corrosion that causes a reduction in fatigue- and fracture- resistance of metal. Hydrogen molecules (blue icons in Figure 2) can dissociate to atomic hydrogen (purple icons in Figure 2) and penetrate a metal. Hydrogen atoms accumulate at stress concentrators such as crack tips or microstructural features like grain boundaries, inclusions, or precipitates. In certain cases, atomic hydrogen can reform as diatomic hydrogen.
Q: What materials are susceptible to hydrogen embrittlement and what are the potential consequences of using the wrong types of stainless steel?
A: Choosing materials that are more susceptible to hydrogen embrittlement can lead to a greater likelihood of a failure in system integrity. Very high-strength materials experience more severe hydrogen embrittlement. Austenitic stainless steels, characterized by their face center cubic (FCC) crystal structure, moderate strength and naturally high ductility, are generally more compatible with hydrogen than many other metals. That said, not all resist hydrogen embrittlement equally. See Figure 3.
While stainless steels are generally more compatible with hydrogen than many other metals, not all resist hydrogen embrittlement equally.
Fatigue capability reduction is a greater concern than ductility loss. Ductility refers to the degree to which the material can sustain plastic deformation under tensile stress before failure. Appropriately designed components are not subjected to stresses that result in macroscopic plastic deformation. Instead, cyclic loading due to pressure cycling, vibration, or other service loading can result in slowly accumulated localized plastic damage and failure due to fatigue—the cracking of the steel due to repeated stresses or loadings. System or component failure potential may become further exacerbated if materials are subject to corrosive environmental factors.
Component failure, of course, can lead to a number of undesirable outcomes, including:
- Potential safety issues
- Excessive downtime for maintenance or repair
- More frequent component replacement
- Sustainability concerns due to hydrogen escaping into the environment
- Higher total cost of operation and ownership for the asset
Q: How do you know if stainless steel is of appropriately high quality for use with hydrogen?
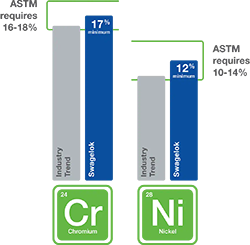
A: High-quality stainless steels containing elevated levels of nickel have been shown to be better suited to handle hydrogen—particularly over a long service life.
As seen in Figure 4, the American Society for Testing and Materials (ASTM) requires a minimum of 10% nickel in 316 stainless steel formulations, but 316 stainless steel with a minimum of 12% nickel is better for the unique challenges posed by hydrogen. Nickel content helps stabilize the microstructure of stainless steel, enabling it to be more resistant to hydrogen embrittlement. In our testing, we have found hydrogen embrittlement’s effect on tensile ductility in 316 stainless steel with 12% Ni was found to be minor.
316 stainless steel with a minimum of 12% nickel is better suited for the unique challenges of hydrogen.
While 316 stainless steel with high nickel content is generally a compelling choice for hydrogen system construction, there may be situations where performance criteria for a specific application, such as a need to prioritize material strength or corrosion resistance, make another material choice a good option. Proper system design and maintenance can help deter embrittlement in these cases. Organizations like Swagelok continue to research and understand the impact of hydrogen on other alloys and can help you make informed decisions.
Q: How do I make sure I choose the right high-performance materials for hydrogen handling?
It can be challenging to discern which materials are optimal for different fluid handling applications, particularly in the burgeoning hydrogen industry. But it is important to get it right. There are long-term implications for specifying materials for hydrogen handling systems. Most importantly, the wrong choices have the potential to damage hydrogen’s reputation as a reliable and viable fuel source for a cleaner future.
Seek out suppliers that can demonstrate a thorough understanding of materials science and have developed products successfully used in hydrogen applications. If you need assistance, Swagelok’s hydrogen specialists are happy to help you select optimal materials for your application and find a solution that meets your needs.
Related Hydrogen Handling Articles

What to Look for in Hydrogen Valves
Valves are critical parts of hydrogen fluid systems. Learn what to look for in an ideal hydrogen valve and how it can contribute to safer, more reliable hydrogen transportation.

The Anatomy of a Hydrogen Fitting
Learn why fittings designed specifically for hydrogen applications can help fuel cell vehicle OEMs and infrastructure developers achieve safer and more reliable fuel systems.

Ask Swagelok: Hydrogen, Sampling and Corrosion Insights
Explore expert insights on fluid systems with Swagelok's video series. Topics include hydrogen design and embrittlement, offshore corrosion, liquid sampling and more.



